

Pipeline
-



Pipeline
JIN-A02
JIN-A02
Target Disease Information
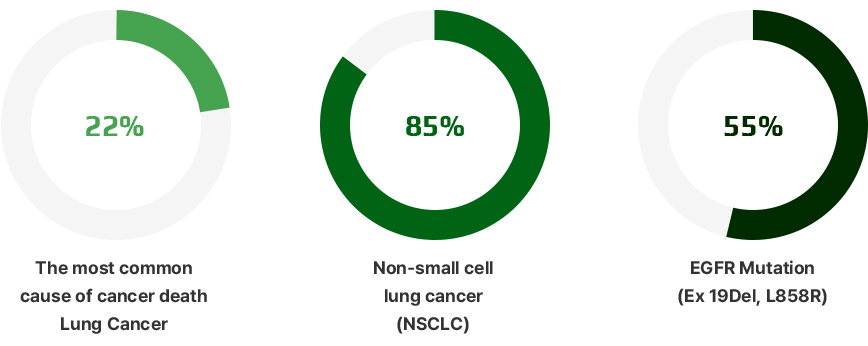
- Lung cancer exhibits the highest incidence and mortality rates among all cancers, and non-small cell lung cancer (NSCLC), which accounts for 85%
of lung cancers, is a representative refractory cancer with low treatment response rates and rapid metastasis. - The epidermal growth factor receptor (EGFR) mutation, a representative driver mutation in NSCLC, is highly prevalent—observed in 40–55%
of Asian NSCLC patients, including those in Korea. - The C797S mutation, which emerges as an acquired resistance mutation following third-generation EGFR-TKI therapies, currently has no treatment options.
Introduction of JIN-A02
- JIN-A02 is a kinase inhibitor targeting EGFR mutations in NSCLC. It exhibits potent anticancer effects against the key acquired resistance mutation EGFR C797S,
which emerges after third-generation EGFR-TKI therapies. Additionally, it demonstrates superior efficacy compared to third-generation EGFR-TKIs against
compound mutations involving C797S—whether in combination with exon 19 deletions, L858R, or T790M—covering both double and triple mutation profiles.
This highlights its potential as a promising next-generation EGFR-TKI. - It also shows high blood-brain barrier permeability and robust intracranial activity, addressing brain metastases—a major metastatic site in NSCLC—and
suggests potential clinical response for brain metastases. - JIN-A02 exhibits high selectivity for mutant EGFR over wild-type EGFR, and maintains very favorable safety profiles even at high doses.
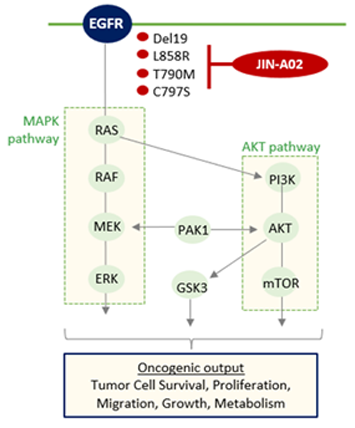
Mechanism of Action of JIN-A02
- Superior efficacy against EGFR exon 19 deletions, as well as L858R, T790M, and C797S mutations
- High selectivity for mutant EGFR compared to wild-type EGFR
- Superior target binding affinity and efficacy compared to third-generation EGFR-TKIs
Key Research Findings of JIN-A02
- Phase 1/2 open-label, multicenter clinical trial evaluating safety, tolerability, pharmacokinetics, and antitumor activity of JIN-A02 in patients with advanced
EGFR-mutant NSCLC(NCT05394831) - Regulatory approvals obtained in Korea (2023), the US (2022), and Thailand (2022).
- No drug-related serious adverse events or dose-limiting toxicities reported to date in enrolled patients.
JIN-A02 Conference Presentation Status
| Conference | Topic |
|---|---|
| 2025 ASCO (Poster presentation) | Clinical |
| 2025 AACR (Poster presentation) | Clinical |
| 2024 ENA (Poster presentation) | Clinical |
| 2024 WCLC (Poster presentation) | Clinical |
| 2024 ASCO (Poster presentation) | Clinical |
| 2024 AACR (Poster presentation) | Clinical |
| 2023 ESMO Asia (Poster presentation) | Clinical |
| 2023 WCLC (Poster presentation) | Clinical |
| 2022 ESMO (Poster presentation) | Preclinical |
| 2022 WCLC (Oral presentation) | Preclinical |
- AACR: American Association for Cancer Research
- ASCO: American Society of Clinical Oncology
- ENA: EORTC-NCI-AACR(EORTC - NCI - AACR)
- ESMO: European Society For Medical Oncology
- WCLC: World Conference on Lung Cancer
National Research and Development Project
- Selection as a project in the 1st phase of the 2025 National New Drug Development Program funded by KDDF


